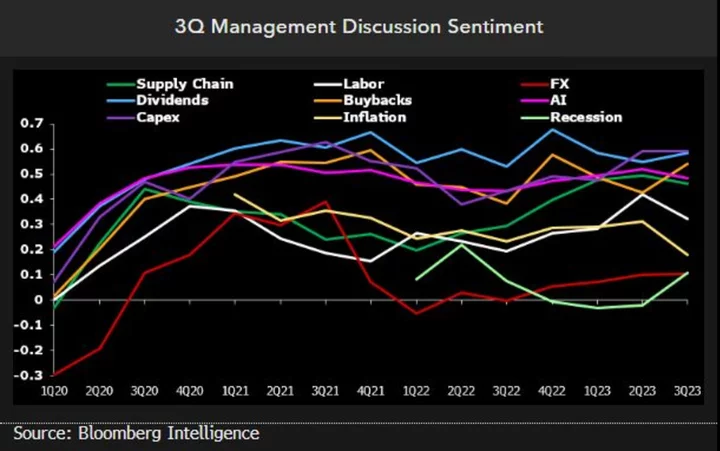
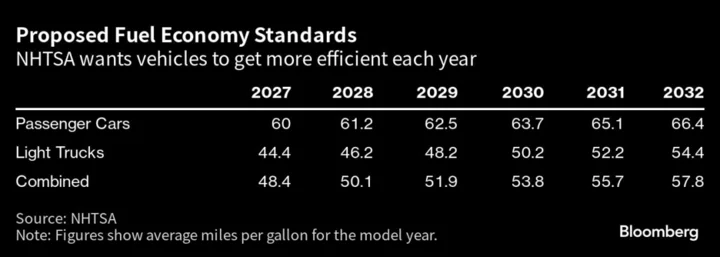
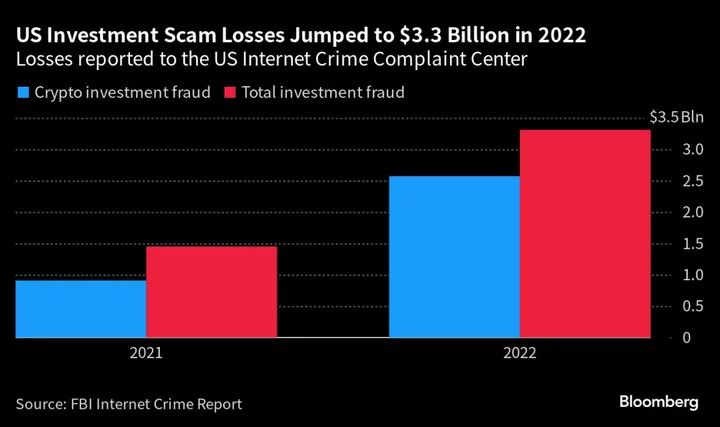
Serum Institute of India Ltd. became the world’s biggest vaccine maker by producing low-cost inoculations for developing nations that other companies wouldn’t. Now it’s looking to tap the rich world’s need for those shots as well.
Over the next three years, Pune-based Serum plans to start production of yellow fever and dengue shots for European and American travelers to countries where those diseases are endemic, Chief Executive Officer Adar Poonawalla said in an interview. That would add higher-margin products to the company’s growing suite of immunizations, which include a sub-$4 malaria shot that was approved by the World Health Organization this week.
Serum, a family-owned business that branched out from racehorse-breeding, has thrived by targeting neglected diseases that plague the world’s poorest places. With its Covid-19 shots and plans for a so-called childhood TDAP vaccine that targets a constellation of ailments like whooping cough, the company is looking to expand in more competitive, and potentially more profitable, markets.
“We’ve got one or two vaccines like the TDAP combination headed for Europe and the US in the next two years,” he said. Serum, India’s most valuable unlisted company, already supplies vaccines to 170 nations, including Covid shots sold in Europe and the US.
While company officials toyed with the idea of going public during the pandemic, when it needed $2 billion to boost production to 4 billion doses a year, there are no plans to do so at the moment, Poonawalla said. The company financed its 2020 expansion for the production of Covid vaccines by taking advances, including from the Indian government and philanthropists like Bill Gates, he said.
Going public “does hinder your decision making,” Poonawalla said. “I would probably have been chastised for charging $3 per shot as a CEO where there was clearly money on the table to charge higher prices.”
The process did come with setbacks. Serum struggled to meet demand and its obligations as the pandemic raged and a factory fire slashed production. In April 2021, the Indian government banned Covid vaccine exports to prioritize the local population. The ban wasn’t lifted until November 2021.
The company could change its approach to take on debt or add partners if a further expansion is required that it can’t finance internally, Poonawalla said.
Containing Costs
Currently, Serum spends $150 million to $250 million a year on improving production and conducting research, out of its $850 million to $900 million in annual revenue, he said. It costs $50 million to $100 million over a handful of years to develop a new vaccine, with a price tag exceeding $200 million once clinical trials and manufacturing are included, he said.
The fiscally conservative approach gives Serum the ability to chart its own course, helping it to roll out meningitis and human papillomavirus vaccines for poor countries. It’s also allowed Poonawalla’s family to amass a fortune. His father, Cyrus, is worth $19.2 billion, according to Bloomberg’s rich list.
Cost is one reason the company doesn’t plan to expand production in Africa, where leaders are clamoring for vaccine factories to be built on the continent, he said. Aside from a facility in the Netherlands, all manufacturing is likely to remain in India where economies of scale help drive down expenses, he said.
“If you start making a product in Africa, the prices are going to go up and we don’t want that to happen,” he said. “We want to keep the prices as low as possible to have the access as wide as possible.”
The company is experimenting with allowing some of its vaccines to be made under a licensing deal, most notably with South Africa’s Aspen Pharamacare Holdings Ltd.
Lessons Forgotten
The expansion in vaccine production necessitated by Covid has better positioned the world to tackle the next pandemic, at least as far as the private sector is concerned, Poonawalla said. Diagnostics and testing capacity is also on firmer footing, he said.
“The private sector will always rise to the occasion, either because it’s a good business opportunity or it is something strategic they want to do,” he said.
What he’s worried about is the slow pace of new product approvals and the reluctance of governments to work together.
“We have not, sadly, made a lot of strides,” he said. “After the pandemic sort of went away, everyone got back to all their other issues and sort of lost focus on the future preparedness.”
--With assistance from Bhuma Shrivastava.