LOWELL, Mass.--(BUSINESS WIRE)--Oct 6, 2023--
RevBio, Inc., announced that it has been awarded a grant from the National Institute on Aging (NIA), part of the National Institutes of Health (NIH), through its Commercialization Readiness Program of up to $3.4 million over the next three years ( 1SB1AG085803-01 ). This grant will enable the company to finish its late-stage product development activities and conduct a clinical trial to demonstrate the safety and efficacy of the product in comparison to the current standard of care.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20231006480237/en/
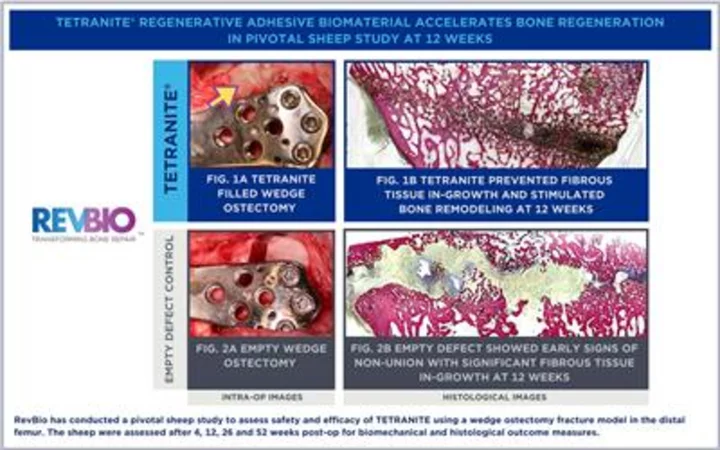
RevBio has conducted a pivotal sheep study to assess safety and efficacy of TETRANITE using a wedge ostectomy fracture model in the distal femur. The sheep were assessed after 4, 12, 26 and 52 weeks post-op for biomechanical and histological outcome measures. (Photo: Business Wire)
The research funded by this grant will focus on the ability for the patented TETRANITE® technology to fill gaps in bone, fixate bone fragments, and accelerate healing through its osteoconductive capabilities for fractures in the extremities. TETRANITE will be used both as an interoperative fixation aid and as an adjunct to traditional hardware fixation to provide immediate load sharing between bone and metal plate and screw systems. By providing additional stability, TETRANITE will help achieve better healing and prevent complications and revision surgeries. It can also be injected percutaneously as a minimally invasive stand-alone method of fixation to surgically treat fractures without the need for conventional open reduction internal fixation (ORIF). This grant will expand upon the pre-clinical research funded by a previous $2 million SBIR Phase II grant from the National Institute on Aging ( R44AG060881-02 ).
“Our pre-clinical studies have demonstrated that TETRANITE is able to redistribute most of the load from plates and screws thereby reducing the likelihood of hardware failure and delayed healing,” said Brittany McDonough, RevBio’s R&D Program Manager, who has led the development of the fracture fixation product. “This adhesive material also provides a resorbable scaffold for the regeneration of bone which accelerates healing within the first few weeks following a traumatic injury.”
Upper and lower extremity injuries equate to more than 40% of the approximate 2.1 million fragility fractures which occur each year in the United States. 1,2 This number is expected to triple by 2040 as the population ages. 3,4 External fixation, percutaneous pinning with K-wires, and conventional ORIF procedures that involve the use of plates and screws are interventions that are currently being used to treat these extremity fractures. 5 However, despite advances in surgical technique and implant design, nonunion, malunion, and hardware failure continue to remain a significant cause of revision surgeries in the elderly. 6 Complication rates as high as 36% have been reported and involve the onset of carpal tunnel syndrome, complex regional pain syndrome, tendon irritation and rupture, and deep infection. 7,8 These complications can lead to difficulty performing basic daily tasks, loss of independence, reduction in quality of life, and increased mortality. 9,10
“Fractures are often comminuted, and it is difficult to get the pieces back together and so having an adhesive would really help achieve an anatomic reduction,” said Michael J. Weaver, MD, Chief of Orthopaedic Trauma at Brigham & Women’s Hospital, and member of RevBio’s Orthopaedic Scientific Advisory Board. “This material is mechanically strong to allow early weight bearing which is something we cannot do currently in peri-articular fractures treated with metal implants. Having a material that can both augment fixation to prevent hardware failure and facilitate healing will fundamentally improve how our patients do.”
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Numbers 1SB1AG085803-01 and R44AG060881-02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
About RevBio, Inc.
RevBio, Inc., is a clinical stage medical device company engaged in the development and commercialization of a patented, synthetic, injectable, self-setting, and osteoconductive bone adhesive biomaterial called TETRANITE®. The company is developing a pipeline of products based on this technology for use in the dental, cranial, and broader orthopaedic markets as well as applications in the animal health market. RevBio's Tetranite technology is not yet approved for commercial use.
_____________________________
1https://www.aaos.org/aaosnow/2022/thursday/research/research12/
2https://www.osteoporosis.foundation/facts-statistics/epidemiology-of-osteoporosis-and-fragility-fractures
3 Watts, Nelson B., et al. "National Osteoporosis Foundation 2008 Clinician's Guide to Prevention and Treatment of Osteoporosis and the World Health Organization Fracture Risk Assessment Tool (FRAX): what they mean to the bone densitometrist and bone technologist." Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry 11.4 (2008): 473-477.
4 Burge, Russel, et al. "Incidence and economic burden of osteoporosis‐related fractures in the United States, 2005–2025." Journal of bone and mineral research 22.3 (2007): 465-475.
5 Grewal R, MacDermid JC, King GJ, Faber KJ. Open reduction internal fixation versus percutaneous pinning with external fixation of distal radius fractures: a prospective, randomized clinical trial. J Hand Surg Am. 2011 Dec;36(12):1899-906. doi: 10.1016/j.jhsa.2011.09.015. Epub 2011 Nov 3. PMID: 22051229.
6 Kloen, Peter, Geert A. Buijze, and David Ring. "Management of forearm nonunions: current concepts." Strategies in trauma and limb reconstruction 7 (2012): 1-11.
7 Thorninger, Rikke, Mette Lund Madsen, Daniel Wæver, Lars Carl Borris, and Jan Hendrik Duedal Rölfing. "Complications of volar locking plating of distal radius fractures in 576 patients with 3.2 years follow-up." Injury 48, no. 6 (2017): 1104-1109.
8 Watts, Nelson B., et al. "National Osteoporosis Foundation 2008 Clinician's Guide to Prevention and Treatment of Osteoporosis and the World Health Organization Fracture Risk Assessment Tool (FRAX): what they mean to the bone densitometrist and bone technologist." Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry 11.4 (2008): 473-477.
9 Marchewka, Jakub, Jacek Głodzik, Wojciech Marchewka, and Edward Golec. "Higher Mortality in Men Compared with Women following Distal Radius Fracture in Population Aged 50 Years or Above: Are Common Distal Radius Fracture Classifications Useful in Predicting Mortality?" BioMed research international 2019 (2019).
10 Nellans, Kate W., Evan Kowalski, and Kevin C. Chung. "The epidemiology of distal radius fractures." Hand clinics 28, no. 2 (2012): 113-125.
View source version on businesswire.com:https://www.businesswire.com/news/home/20231006480237/en/
CONTACT: Michael Tiedemann
mtiedemann@revbio.com
KEYWORD: MASSACHUSETTS UNITED STATES NORTH AMERICA
INDUSTRY KEYWORD: MEDICAL SUPPLIES BIOTECHNOLOGY FDA OTHER HEALTH HEALTH PHARMACEUTICAL OTHER SCIENCE RESEARCH SURGERY SCIENCE CLINICAL TRIALS
SOURCE: RevBio, Inc.
Copyright Business Wire 2023.
PUB: 10/06/2023 02:19 PM/DISC: 10/06/2023 02:20 PM
http://www.businesswire.com/news/home/20231006480237/en